Україна
Ми працюємо в Україні з 2004 року. Наша команда глибоко розуміє важливість належного догляду за пацієнтами під час клінічних досліджень. Ми маємо унікальний досвід ефективної роботи як на українському ринку, так і за його межами.
Графік вихідних днівІндивідуальний підхід до кожного клієнта
Склад працює з урахуванням специфіки кожного клієнта — ми постійно вдосконалюємо процеси та послуги, щоб співпраця з нами була максимально зручною й ефективною.
Швидка доставка біозразків
Доставка біозразків по всій Європі за 48–72 години
Два ліцензовані склади
Ми маємо сертифіковані склади, що повністю відповідають вимогам до безпечного зберігання препаратів, призначених для клінічних досліджень
Контактна інформація
Адреса офісу Вул. Антоновича, 172, БЦ «Палладіум Сіті» (11 поверх), Україна, Київ, 03150 Телефон +380 44 498 58 76 Факс +380 44 498 58 77 Адреса основного місцевого складу Вул. Колгоспна, 4, Крушинка, Київська область, 08635, Україна Адреса регіонального складу Вул. Польна, 6, м. Броди, Львівська область, Україна
Інша інформація
Площа складу Основний склад: 2754,1 м2 Регіональний склад: 511 м2
- Хаб ЄС: Угорщина
- Боснія і Герцеговина
- Хорватія
- Данія
- Грузія
- Ірландія
- Молдова
- Чорногорія
- Північна Македонія
- Сербія
- Велика Британія
- Україна
- Єгипет
- Ізраїль
- Саудівська Аравія
- Туреччина
- Хаб Африки південніше Сахари: Кенія
- Південна Африка
- Канада
- Домініканська Республіка
- США
- Аргентина
- Бразилія
- Чилі
- Колумбія
- Коста-Рика
- Гватемала
- Панама
- Австралія
- Китай
- Індія
Послуга, що надається
Послуга, що надається за запитом
Тип складу
Хаб ЄС: Угорщина
Ірландія
Боснія і Герцеговина
Хорватія
Грузія
Молдова
Чорногорія
Північна Македонія
Сербія
Велика Британія
Україна
Данія
Центральні склади Oximio
Партнерська мережа Oximio
Види матеріалів
Антибіотики
Біологічні матеріали
Препарати крові та кровозамінники
Контрольовані лікарські засоби за запитом
Цитотоксичні препарати
Небезпечні речовини
Лікарські засоби, що містять генетично модифіковані клітини
Радіоактивні речовини
Супутні матеріали/Обладнання
Лікарські засоби на основі медичного канабісу
Температурні режими
Неконтрольовані температурні умови
Температура +15°C до +25°C
Температура +2°C до +8°C
Низька температура -25ºC до -15ºC
Ультранизька температура -80ºC до -60ºC
Рідкий азот (-196ºC)
Ліцензії
Сертифікат GDP
Сертифікат GMP
ISO9001:2015
Сертифікат MIA
WHL ліцензія
Ліцензія на діяльність з обігу наркотичних засобів, психотропних речовин і прекурсорів
Послуги
Маркування (JIT)
Закупівля компараторів
Імпортер
DTP (безпосередньо до пацієнта)
DTS (доставка безпосередньо до клінічного центру)
Поточна географія присутності в Україні
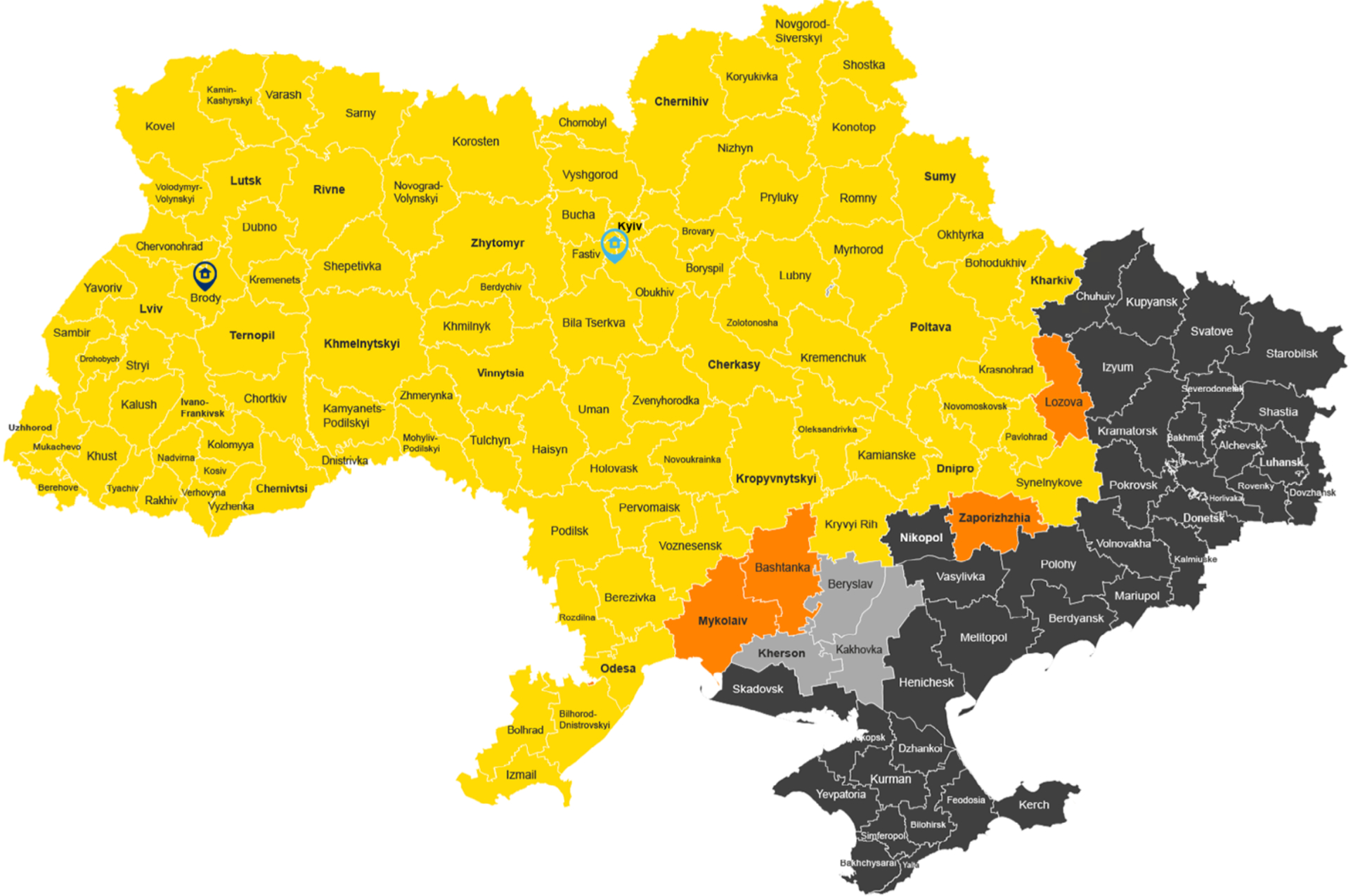


Робота у звичному режимі
У цих регіонах надання транспортних та додаткових послуг (пряма доставка пацієнтам, медична допомога вдома, транспортування пацієнтів) здійснюється у звичному режимі.
Розглядається в індивідуальному порядку
Питання надання транспортних і додаткових послуг (пряма доставка пацієнтам, медична допомога вдома, транспортування пацієнтів) у зазначених регіонах/містах розглядається в індивідуальному порядку.
Можливо після оцінки
Наразі стандартні послуги тимчасово недоступні. Надання додаткових послуг (пряма доставка пацієнтам, медична допомога вдома, транспортування пацієнтів) можливе після попередньої оцінки.
Надання послуг призупинено
Надання всіх послуг у цьому регіоні тимчасово призупинено.
Latest updates: Останні оновлення: 22 травня
Транспортні послуги:
Сумська та Роменська громади переведені до жовтої зони. Звертаємо вашу увагу, що ця інформація є актуальною на вказану дату та може змінюватися без попередження. У разі запитань просимо звертатися за цим посиланням.
Україна
Розташування складу
Перегляньте добірку корисних посилань. Щоб дізнатися більше про регіон, оберіть відповідне посилання.
Корисні посилання щодо України
Останні законодавчі акти Міністерства охорони здоров’я щодо клінічних випробувань
ПереглянутиНаказ про затвердження Порядку проведення клінічних випробувань лікарських засобів та експертизи матеріалів клінічних випробувань і Типового положення про комісії з питань етики
ПереглянутиКорисні посилання щодо України
Закон про внесення змін до деяких законів України щодо державного регулювання обігу рослин роду коноплі (Cannabis) для використання у навчальних цілях, освітній, науковій та науково-технічній діяльності, виробництва наркотичних засобів, психотропних речовин та лікарських засобів з метою розширення доступу пацієнтів до необхідного лікування
ПереглянутиНаказ про затвердження Порядку затвердження та проведення програми розширеного доступу пацієнтів до незареєстрованих лікарських засобів та програми доступу суб’єктів дослідження (пацієнтів) до досліджуваного лікарського засобу після завершення клінічного випробування та Змін до Порядку ввезення на територію України незареєстрованих лікарських засобів, стандартних зразків, реагентів
ПереглянутиХочете дізнатися більше?
Завантажуйте брошуру Oximio для отримання додаткової інформації
Завантажити брошуру