Global Clinical Trial Logistic Solutions
Our comprehensive global clinical trial supply logistics have been designed to provide a flexible, all-in-one solution to fulfil your individual requirements from start to finish
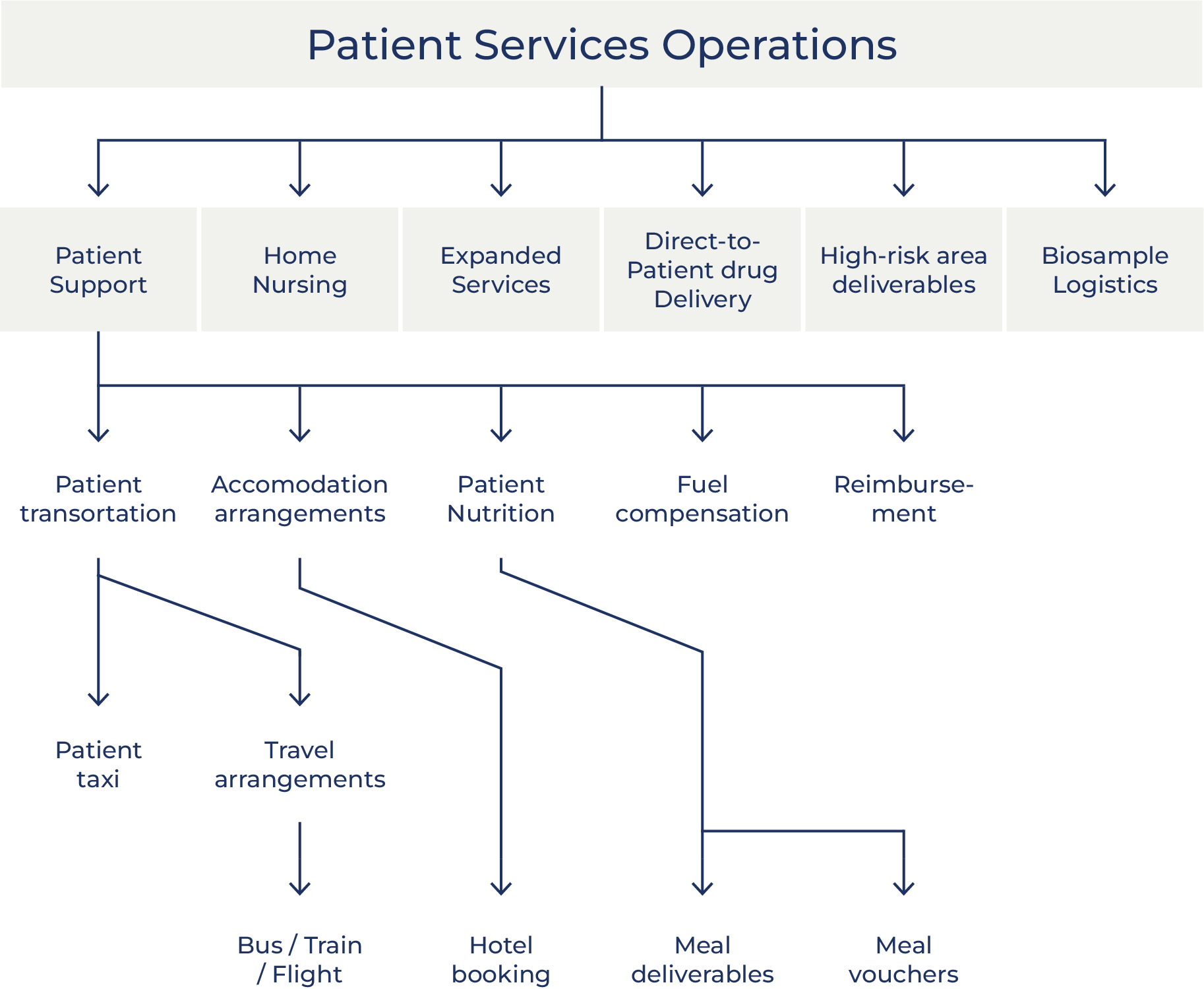
Oximio´s Patient Solutions
01.
Project Management
Project Management
Effective clinical trials project management is crucial for ensuring the successful and timely completion of research studies. We understand that navigating the complexities of clinical trials can be challenging and requires expert industry knowledge. With a variety of procedures, regulations and language barriers to deal with, our team of experienced project managers will provide comprehensive support through every stage of the process.
A clinical trial plan that’s right for you
Running a clinical trial requires stringent planning, organisation and co-ordination. A smooth supply chain delivered by solid project management is essential throughout to guarantee the smooth running of the trial. Our clinical trial project management service is designed to give you the best value for money, with flexible pricing.
View the options below and choose a plan that meets your budget.
Set-up:
Management:
client needs
Licenses:
Clearance support:
Import support:
Export support:
Closure:
Need more info?
02.
Customs Clearance & IOR/EOR
03.
Depot & Distribution
Cold Chain Services
Include:
Leading Storage Capacities in the following temperature-controlled conditions:
IRT Interaction and Central
Inventory Management24/7 security
Variety of cold shipper solutions and data-loggers
State-of-the-art temperature and humidity monitoring
ReguBack-up power sources
Own local couriers
04.
Return Plan & Destruction
Clinical Return Plan
During the study start-up, we proactively develop a clinical return plan taking into consideration drug or country-specific challenges. Oximio’s project management team takes care of return management throughout the life of your study to avoid any backlog at investigational sites and performs drug reconciliation at the desired accountability level (either shipper, kit or pill).
We always consider site capacity and provide the necessary packaging materials (cardboard boxes, sharp-bins, bubble wrap) for returns handling.
By pooling the returns into bulks at local and regional levels we are removing the financial burden from the sponsor and streamlining trial closeout.
05.
Bio-Samples Management
Bio-samples Management
As an established logistics provider with expertise dedicated to clinical trials, Oximio provides greater efficiency and control for the collection and return of your biological samples, while minimising risks to safety.
With Oximio’s bio-sample management shipment service, you can expect:
Quick and comprehensive administrative support for document management during the export of bio-samples
Highly reliable temperature-controlled packaging
Support for a range of temperature ranges across ambient, refrigerated, frozen and dry ice
Door-to-door service – from site to the central lab
Solutions to overcome any adverse road or infrastructure conditions
Experts in the most efficient export routes
Regulatory and customs clearance expertise
Proven implementation of solutions during military conflict and other public emergencies
Bio-sample Logistics Workflow
- The PI completes a collection request form (the form, as well as other export document templates, including a letter to customs and a proforma invoice, are prepared by Oximio in advance as part of the project setup)
- Oximio collects the bio-samples from the site (including controlled packaging, temperature loggers and related materials – ambient, refrigerated frozen or dry ice).
- Oximio undertakes the export procedures
- Oximio delivers to the central lab
- Oximio provides P.O.D
Note: As the first organisation to resume operations in Ukraine since the start of the crisis, Oximio’s services offer a comprehensive solution that puts shipment integrity and staff safety first.
06.
QP Certificate & Release
07.
Customs Bonded Facilities
Bonded Warehouse Solutions for Clinical Trials
Our bonded and non-bonded warehouses provide:
Regulatory Compliance:
Oximio’s customs bonded and non-bonded warehouses adhere to strict rules and regulations set by customs authorities. They undergo regular inspections, have enhanced security measures, and employ trained teams to handle controlled substances and sensitive pharmaceutical products. These measures ensure compliance with applicable regulations, such as Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP), thereby promoting product integrity and patient safety.Traceability and Record-Keeping:
We maintain meticulous records and provide transparent documentation throughout the storage and distribution process. This ensures full traceability, allowing researchers and regulatory bodies to track and verify the movement of pharmaceuticals at each stage of a clinical trial. Comprehensive record-keeping also facilitates efficient inventory management, simplifies audits, and minimizes the risk of regulatory non-compliance.Efficient Logistics and Inventory Management:
Our warehouses provide dedicated facilities for the handling, storage, and distribution of clinical products. Their specialised infrastructure, along with robust logistics capabilities, enables efficient management of inbound and outbound shipments, reducing the risk of product loss or delays.Temperature Control:
Our custom bonded and non-bonded warehouses can offer specialist temperature control solutions such as ultra-low temperature freezers or cryogenic storage facilities assuring the quality of samples and medicines at all times.
Additional benefits:
Enhanced Security and Compliance:
Our facilities offer heightened security measures, including access control, video surveillance and alarm systems. These measures protect valuable investigational products and minimize the risk of theft, tampering, or unauthorised access. Moreover, strict compliance with regulations ensures that clinical trial supplies meet the required quality standards, safeguarding the integrity of research outcomes.Customs Bonded Warehouse
- Cross Border Shipments
- Bulk shipments to the warehouse with lower number of
intercontinental flights - More efficient manageable sized supplies, re-supplies.
Experienced Courier Partners’ Network
- Reduced risk
- Improved Supply Chain timelines
- Local couriers with the lower shipment prices
Attract more Clinical Trials
- New technologies
- Specific Temperature Control requirements
- Cell and Gene Therapies
08.
Courier Services
Courier Services
Our medical delivery couriers are exclusively dedicated to pharmaceutical transportation, ensuring utmost care and attention. To safeguard your valuable products, our delivery vehicles are equipped with temperature-controlled systems, monitored in real-time through GPS tracking.
Discover the flexibility and agility of our medical courier services, tailored to meet your specific requirements.
Our worldwide depot network
Our global network of depots and warehouses are strategically distributed across MENA, LATAM, APAC, Europe, Africa, and North America, ensuring an agile supply chain for clinical trials whilst maintaining the highest operational standards.
Oximio Regional Map
We work according to the demands, logistics and regulations of each market around the world.
To get more local information, please click on the country of your interest.
- Oximio’s Central Depots
- Oximio’s Customs Bonded Depots
- Oximio’s Partner Depots