Ukraine
Established in 2004, our Ukrainian team have a strong understanding of the critical importance of patient care in clinical trials, we have unparalleled proficiency in navigating the complexities of the Ukrainian market and wider market beyond
Holiday ScheduleFlexible customer approach
Depot able to adapt to client needs, improving services and processes to get the most out of the experience with us
Fast Bio Samples Delivery
48-72 hours Bio Sample delivery service throughout the European territory
Two WHL licenced
warehouses for clinical trials certify our high quality standards of storage
Contact information
Office address
172 Antonovycha str, BC Palladium city (11 floor), Ukraine, Kyiv, 03150
Phone
Fax
Main local depot address
4 Oleny Telihy Str., Krushynka village, Fastiv district, Kyiv region, Ukraine
Regional depot address
6 Polna str., Brody city, Lviv region, Ukraine
Other information
Depot surface
Main depot: 2754,1m2
Regional depot: 511m2
Service offered
Service offered upon request
Depot type
EU Hub: Hungary
Ireland
Bosnia & Herzegovina
Croatia
Georgia
Moldova
Montenegro
North Macedonia
Serbia
UK
Ukraine
Denmark
Oximio’s Central Depots
Oximio’s Partner Depots
Products Managed
Antibiotic
Biological Goods
Blood Product or Derivative
Controlled Drugs on Request
Cytotoxic
Dangerous Goods
Genetically Modified Organism
Radioactive
Ancillary Supply/Equipment
Medicinal Cannabis Products
Temperature Conditions
Ambient (non-controlled)
Ambient (+15°C to +25°C)
Refrigerated (+2°C to +8°C)
Frozen (-25ºC to -15ºC)
Deep Frozen (-80ºC to -60ºC)
LN2 (Cryogenic)
Certifications
GDP License
GMP Certification
ISO9001:2015
MIA Certification
WHL License
Controlled Drugs License
Services
Labeling (JIT)
Comparator sourcing
IOR
DTP (direct to patient)
DTS (direct to site)
Current coverage in Ukraine
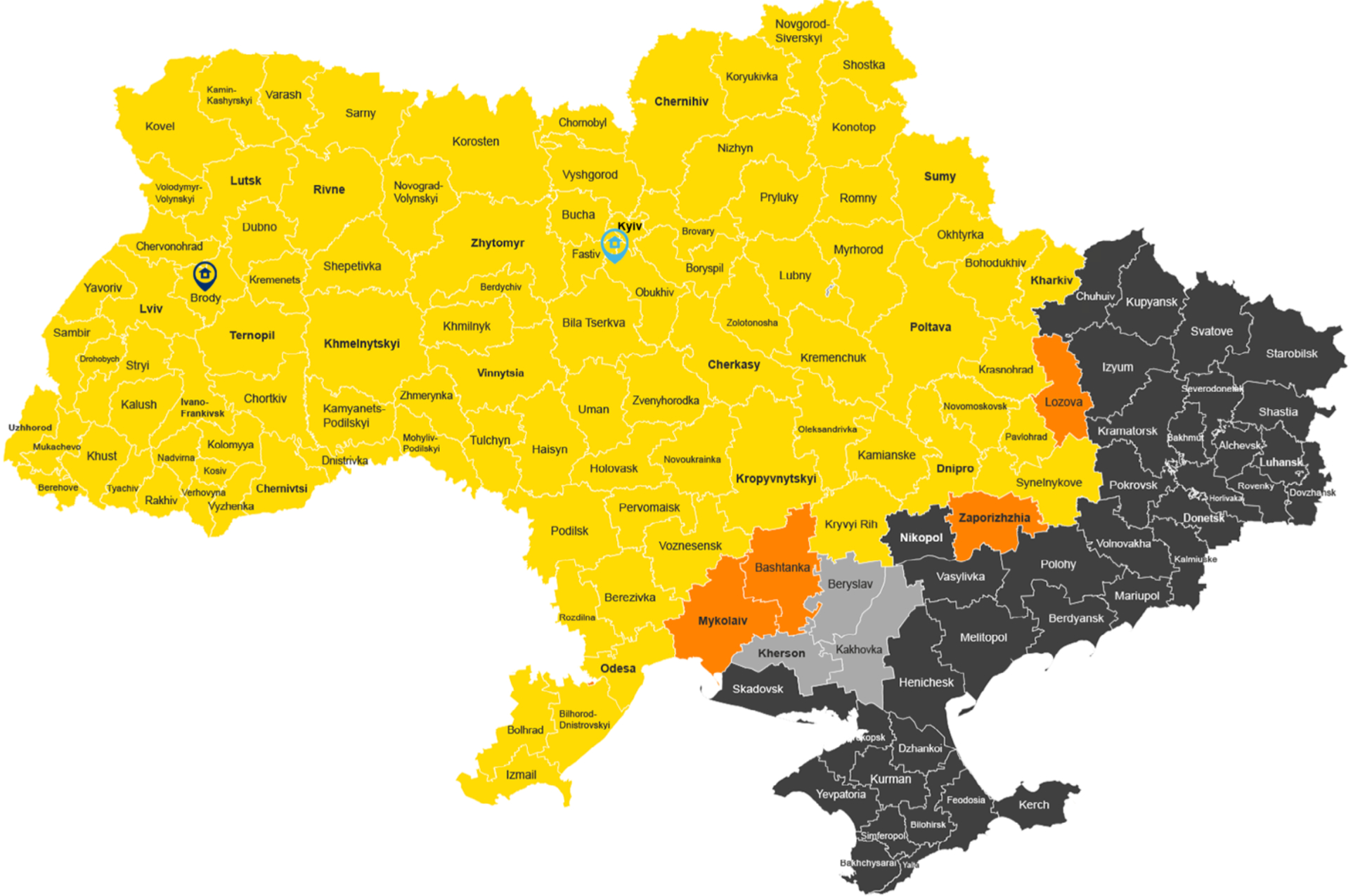


Operating regularly
Transport and extended services (direct-to-patient, home healthcare, patient travel) within this areas are established and operating regulary.
Case-by-case reviewed
Transport and extended services (direct-to-patient, home healthcare, patient travel) to these areas/cities are feasible, but should be reviewed on a case-by-case basis.
Possible with assessment
Standard services are not available. Extended services (direct-to-patient, home healthcare, patient travel) are possible with assessment.
Service suspended
All services in this area are currently suspended.
Latest updates: Latest updates: 22th of May
Transport services:
Sumy, Romny regions are moved to the yellow zone
Please note that this information is accurate for the day indicated, and is subject to change without notice. Please reach out to us with any questions you may have here.
Ukraine
Depot Location
Here you will find useful links. To get local information, please click on the link of your interest.
Ukraine useful links
Latest Ministry of Health legislations for Clinical Trials
View hereLegislation on the approval of the Procedure for conducting clinical trials of medicinal products and examination of clinical trial materials and the Standard Regulation on ethics commissions
View hereUkraine useful links
Law on Amendments to Certain Laws of Ukraine Concerning State Regulation of the Circulation of Cannabis Plants for Use for Educational Purposes, Educational, Scientific and Scientific-Technical Activities, Production of Narcotic Drugs, Psychotropic Substances and Medicines in Order to Expand Patients' Access to the Necessary Treatment
View hereLaw on the approval of the Procedure for the approval and implementation of the program of extended access of patients to unregistered medicinal products and the program of access of research subjects (patients) to the researched medicinal product after the completion of the clinical trial and Amendments to the Procedure for importing unregistered medicinal products, standard samples, and reagents into the territory of Ukraine
View here