Supplementary clinical trial solutions
Oximio launches new services to support sites and investigators affected by the war in Ukraine
Drug accountability and reconciliation
- Recounted and verified returns
- Once collected, returns are recounted at our depot to drug level
- Fully trained employees provided with access to necessary equipment
Clinical trial storage archive
- Transportation and secure storage of site archive data
- Material stored in GxP compliant, temperature-controlled environments
- Stored within guarded, secure depots in central or Western Ukraine
Clinical research printing
- One-stop printing service
- Includes printing of items such as study newsletters, patient booklets and monitoring plans
- Direct deliveries of training materials to clinical sites
Drug accountability and reconciliation: the process
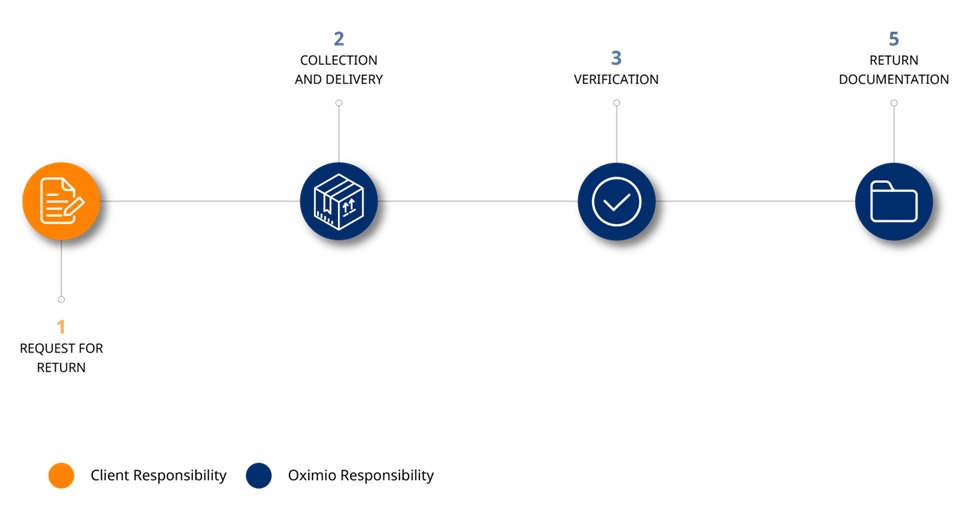
Process description:
- Sponsor/site/CRO requests return of the product
- We collect the shipment from the site and delivers it to one of our depots
- Product is verified at drug level and according to sponsor requirement
- Verified return documentation, confirming the quantity of returned tablets, capsules, vials or other products is produced
Clinical trial archive storage: the process
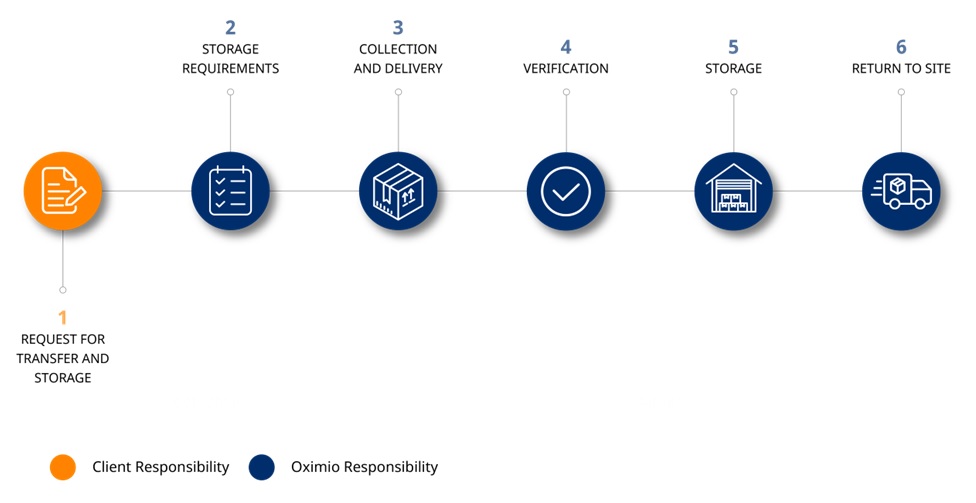
Process description:
- Sponsor/site/CRO requests transfer and off-site storage of the site archive
- We establishe storage requirements, in collaboration with client
- Archive is collected from the site, and delivered to an Oximio depot
- Verification at box level (unless otherwise agreed) is performed
- Archive is stored in temperature-controlled conditions
- Archive is returned to site for audit or monitoring purposes, as required
Clinical research printing: the process
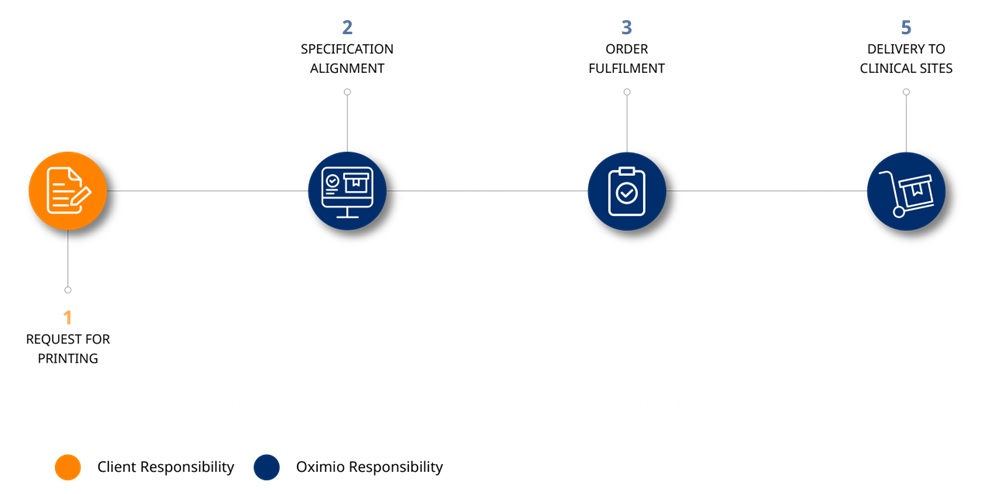
Process description:
- Request from sponsor/site/CRO to print materials relating to the study (materials include, but are not limited to: newsletters, investigator folders, regulatory binders, protocols, subject cards, patient booklets, study reference manuals, ID cards, eCRF completion guidelines, monitoring plan)
- We gather design, layout and quantity requirements
- Order is fulfilled
- We deliver materials to clinical sites
Contact us to discuss solutions for your trials in Ukraine: communications@smo-group.com
